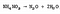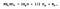The Chemistry of the Beirut Tragedy
The use of ammonium nitrate in explosives is nothing new. It is a potent oxidizing agent that accelerates the combustion of fuels, instantaneously liberating the energy within their bonds. This is how explosives work. The Oklahoma City bombing in 1995 is an example of its devastating potential. A rental vehicle filled with about 4,000 pounds of ammonium nitrate fertilizer prills (1–2 mm soluble beads) treated with diesel fuel (47:1) was detonated in front of the Alfred P. Murrah Federal Building, destroying the adjacent building and damaging 325 others in a substantial radius of the blast.
Ammonium nitrate is a readily-available fertilizer, as plants thrive on the nitrogen present in both the ammonium and the nitrate moieties of the compound. Malicious use has decreased with stiffened regulations and availability.
The use of ammonium nitrate to accelerate the combustion of fuel is common in industrial applications such as mining. The mixture is referred to as ANFO for ammonium nitrate and fuel oil. The actual detonation is a result of instant release of the energy within the fuel’s bonds, driven by the presence of ammonium nitrate.
But ANFO is a relatively slow burn. The explosive power is generated from the rapid formation of gases in a confined space leading to release.
The violent reaction witnessed in the Beirut tragedy is at first difficult to reconcile. You can actually put out a fire with ammonium nitrate — it is a relatively stable compound that does not simply ignite on its own. So while here was 2750 tons (>2.5 million kg) of ammonium nitrate in the warehouse in Beirut, there was no “fuel” component. So how does negligent fertilizer storage translate to a massive tragedy?
Analysis of the video shows a burning fire nearby, and some reports indicate that this started because a welding crew was repairing a gap in the warehouse that set of fireworks adjacent to the ammonium nitrate. Insert question mark here.
In the video you can see the fire burning, with small aerial bursts consistent with fireworks on site.
While relatively stable at room temperature, ammonium nitrate decomposes with heat. It is the degree of heating that dictates the products of the reaction. Below ~300°C ammonium nitrate breaks down into nitrous oxide (N<sub>2</sub>O) and water (H<sub>2</sub>0), plus the reaction releases heat. Neither of these compounds has much consequence under slow decomposition.
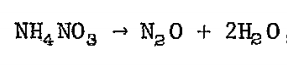
At high temperatures the reaction is much different. The reaction produces gaseous nitrogen (N2) and oxygen (O2) along with water in a massively exothermic (heat producing), rapid release. This is what likely happened in Beirut. The high heat from this preliminary fire led to the rapid chain reaction decomposition of ammonium nitrate that instantaneously ignited the entire load, an explosion 1/5 of Hiroshima. That rapid decomposition led to the tremendous shock wave that broke windows miles away and was heard as far away as Cyprus.
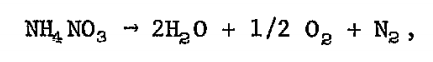
The evidence of ammonium nitrate decomposition in Beirut is also noted by the signature red gas cloud, an indicator of NO2 (nitrogen dioxide) formation.
It is also possible that the stored ammonium nitrate was already decomposing from years of improper storage, and could very well have gone through other transformations from moisture or if it was contaminated with fuels or other combustible materials. The circumstances may have hastened the reaction from the initial fire.
Many of us grew up milling gunpowder or creating model rocket engines, and nitrate-based oxidation compounds were something we always understood and respected. However, their use as principle components of a violent explosion is not immediately clear. Hopefully this short article provided that clarity.
Beirut is no stranger to violent explosions, as decades of civil wars and bombing from Israel have left a lasting impact on the population and its infrastructure. As of this writing the death toll stands as 78. However, there is no chance that this number will remain constant, as the ultimate tragedy of this event will probably take weeks to realize.